[Linked in] Feb 24th K-BPR : Existing biocidal substances registration
Hello, we are Safety Assessment Solution Co., Ltd.
From today, we are going to share about the K-BPR, which stands for Korea Biocidal Product Regulation, enforced since 2019. Among the four obligations (1.Existing biocidal substances registration, 2.Approval of biocidal substances, 3.Approval of biocidal products, 4. Safety and marking standards for products treated with biological substances), we would like to take a quick look around at the first one, "Existing biocidal substances registration".
The target for reporting existing biological substances is the biological substances contained in products distributed in domestic (Korea) before December 31, 2018. The declaration has already been closed by June 30, 2019, through the IT-system, and an approval application plan has been submitted by December 31, 2020. After submitting the application plan, approval will be completed within the grace period for each group (up to 10 years). Refer to the card attached for specific types of biocidal products.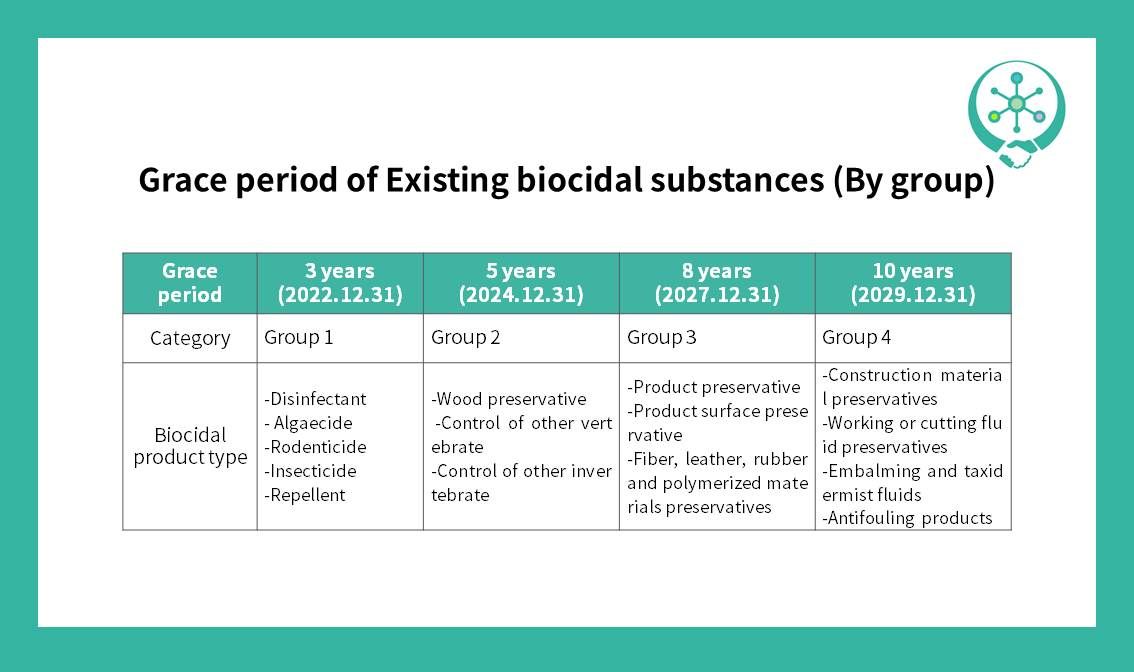
The next week will be the guidance for approval of biocidal substances and products. Any inquiries related to K-BPR, please feel free to contact info@safety-as.com or +82-27540600. Have a great day!
▶ Join our group and get the recently news!: https://www.linkedin.com/groups/13911952/

