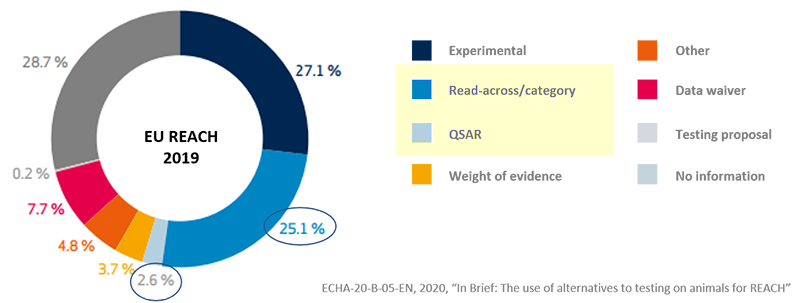
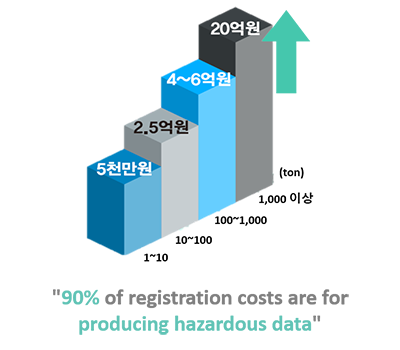
- The production of hazardous test data required for regulatory
response registration takes enormous costs and time. - Read-Across and QSAR data submission services that can be used as
registration data.
(Basis : Article 14 K-REACH and Article 13 (3) of the Enforcement Decree of the K-REACH - By producing and submitting in-silico data instead of actual test data,
- Cost reduction in generating hazard data for regulatory response
(approximately 30%)
- Helping companies develop new substances - Data production only with structural formula without actual test substances
- Useful for 'screening before synthesizing substances' and 'pre-evaluation of substances that are difficult to purify'
QSAR
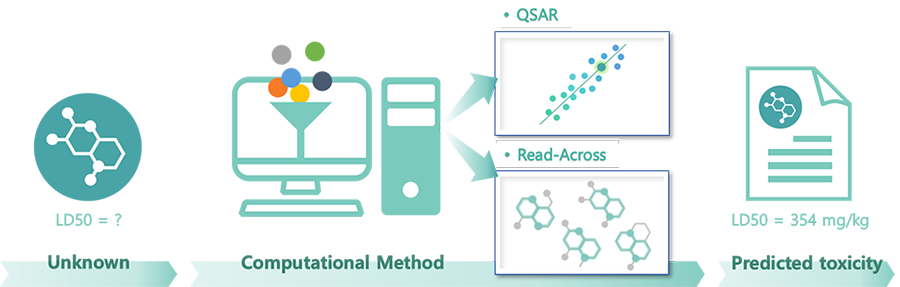
QSARis an abbreviation for Quantitative Structure-Activity Relationship which literally uses the relationship between structure and activity in predicting the properties and toxicity of chemicals. Chemicals with similar structures have the same approach as the existing Read-Across in that they presuppose that they have similar properties, but QSAR uses a model that embodies them and calculates them numerically.
The research is already actively conducted overseas, such as the United States and Europe, and in particular, the EU REACH officially recognizes the use of QSAR data in evaluating the hazards of chemicals. In Korea, guidelines are disclosed and legalized accordingly , so we will actively review the data based on them.
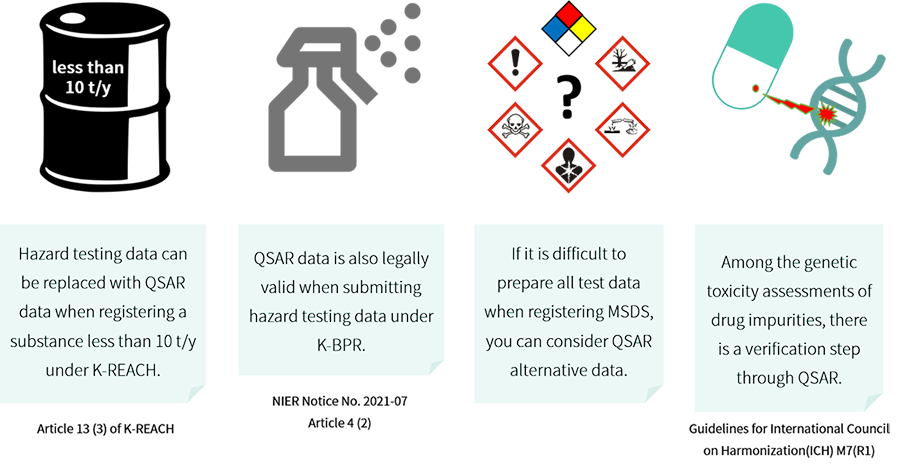
“A chemical substance whose human health or environmental hazards may be determined based on the results obtained from internationally recognized qualitative or quantitative structure activity relationship models (QSAR models), the quantity of which to be manufactured or imported is less than 10 tons per year”
- Article 13 (3) of the Enforcement Decree of the K-REACH



the U.S. Environmental Protection Agency (EPA)
are introducing QSAR data as official
submission data
The use of QSAR(non-test data)
Europe registered at least one out of four as alternative data for exams