[Linked in] Mar 10th K-BPR : Approval criteria of biocidal substances and products
Hello, we are
Safety Assessment Solution Co., Ltd.
The last news regarding K-BPR will be about the Approval criteria of biocidal
substances and products. Specific
approval criteria for safety, effectiveness, efficacy, etc. were prescribed for
determining whether or not to approve a biological agent in accordance with the
K-BPR. (Check below and the attached card.)
1) Do not negatively affect the health or environment of people or animals (Hazardous,
Risk)
2) The effectiveness and effectiveness of
removing harmful substances shall be sufficient.
3) Do not make harmful organisms resistant to
the disease
4) Do not cause unnecessary pain in
vertebrates
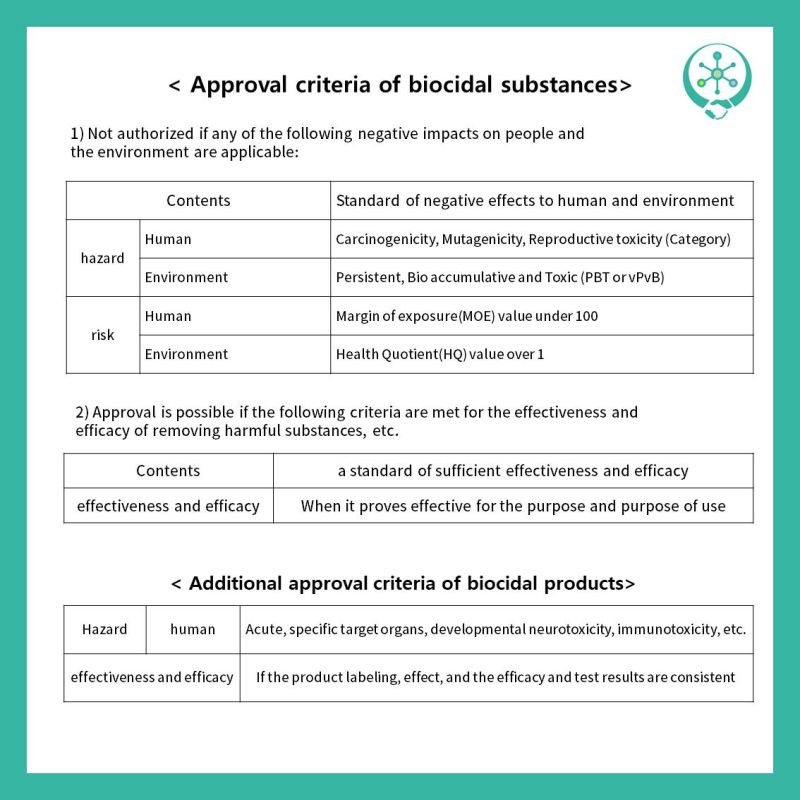
Approval is not possible if there is any negative impact on people and the
environment, and approval is possible if both effectiveness and efficacy are
satisfied.
Also, for product approval, the material
standard and product specific criteria must be satisfied at the same time, and
if the hazardous criteria are not met, the end user (consumer) will not be
approved when determining whether to approve it.
Please check the contents of the attached card
for additional approval criteria in the biological substance and product.
If you have any inquiries related to K-BPR, please feel free to contact info@safety-as.com or +82-27540600. Have a great day!
▶ Join our group and get the recently news!: https://www.linkedin.com/groups/13911952/
